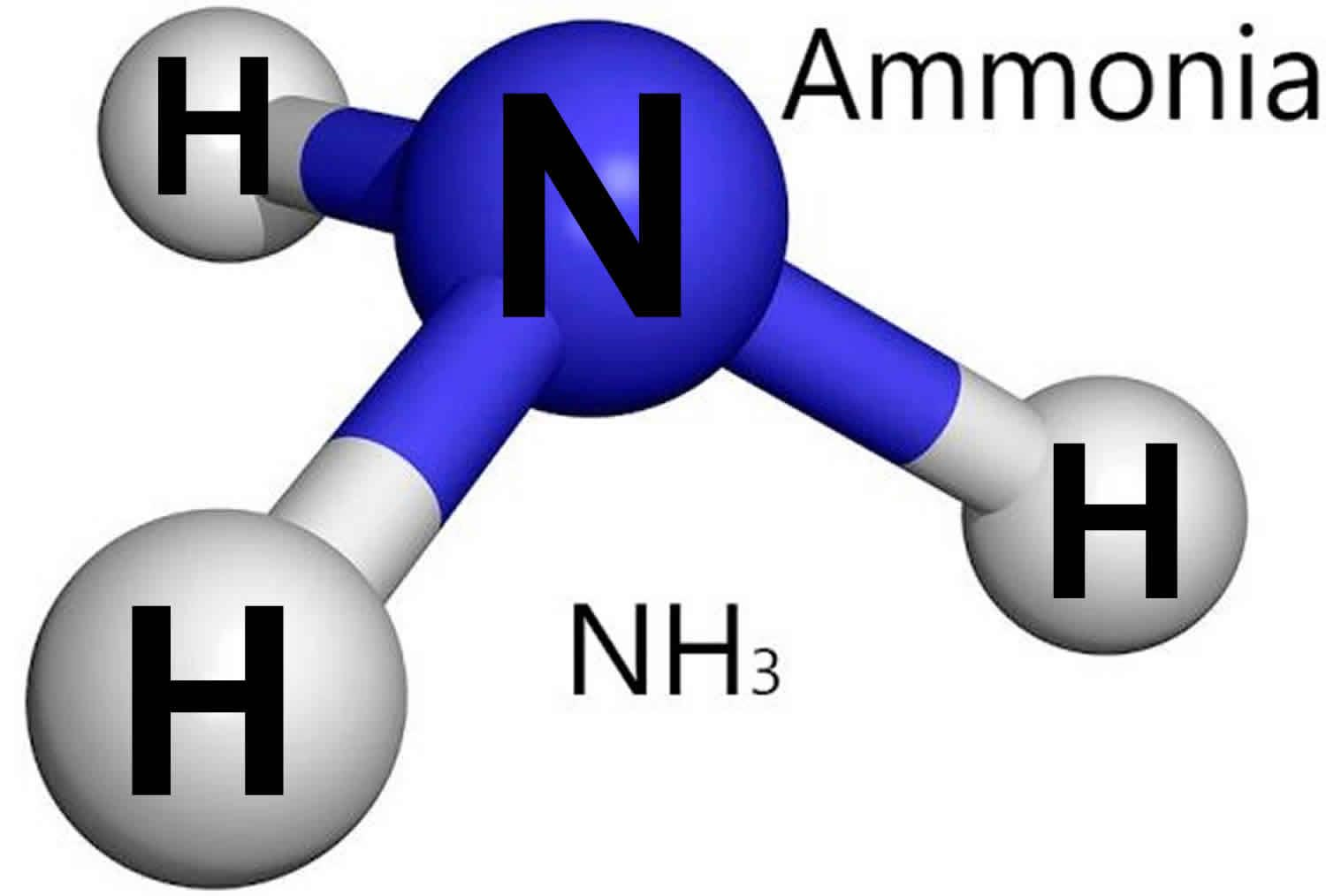
Ammonia holds significant importance as a versatile chemical compound, finding applications in numerous industries and everyday products like fertilizers, cleaners, and refrigerants. In this article, we’ll explore different methods of ammonia production and delve into the specifics of the renowned Haber-Bosch process, which plays a crucial role in generating this essential compound. Through this article, embark on a journey to understand the fascinating world of ammonia production.
Applications of Ammonia
Ammonia finds a wide range of applications in both industrial and commercial settings. Let’s explore some examples:
- Fertilizers: It plays a crucial role in many fertilizers, providing essential nitrogen to promote plant growth, making it an indispensable component for agriculture.
- Refrigeration: Its remarkable refrigerant properties make ammonia a popular choice in industrial and commercial refrigeration systems, keeping things cool efficiently.
- Nitric Acid Production: It serves as a vital ingredient in the production of nitric acid, a versatile chemical used in explosives, plastics, and medicines, among other applications.
- Food Processing: In certain cases, ammonia is utilized as a preservative in food processing due to its ability to eliminate bacteria and extend the freshness of food products.
- Cleaning and Sanitizing: With its potent cleaning properties, this chemical compound is a common ingredient in various cleaning products and disinfectants, making it an effective ally in maintaining hygiene.
- Organic Chemistry: In the realm of organic chemistry, ammonia acts as a significant reagent in the synthesis of various organic chemicals like aniline and ammonium chloride, contributing to the production of diverse compounds.

The diverse applications of this compound makes it a valuable and versatile compound in various industries and daily life.
Ammonia Production Processes
There are several main methods for ammonia production, each with its unique approach:
- Haber-Bosch Process:
The widely adopted method involves the reaction of natural gas with air at high temperatures and pressures. This chemical reaction yields ammonia and water, making it the most commonly used approach for ammonia production. - Production from Biomass:
An alternative to using natural gas involves utilizing biomass, like organic waste or biogas, as a feedstock for ammonia production. This eco-friendly approach reduces reliance on fossil fuels and promotes sustainability. - Production from Ammonium Nitrate:
Ammonium nitrate, a natural fertilizer, can serve as a feedstock to produce ammonia. The process entails the oxidation of ammonium nitrate using sulfuric acid and heat. - Production from Nitrogen and Hydrogen:
Another method involves the reaction between nitrogen and hydrogen, carried out at high temperatures and pressures, utilizing an iron catalyst to facilitate the process.
These diverse methods of ammonia production cater to various industrial needs and contribute to the widespread availability of this crucial compound across different sectors.
Haber-Bosch Process of Ammonia Production
Ammonia production on a large scale primarily relies on the renowned Haber-Bosch process, developed in the early 20th century. This method involves a chemical reaction between natural gas and air under high temperatures and pressures, resulting in the production of ammonia and water.
The essential components and equipment required for the Haber-Bosch process include:
- Natural Gas: Sourced from hydrocarbons found in the earth, natural gas is purified to eliminate impurities and contaminants, serving as a crucial feedstock for ammonia production.
- Air: Comprising mainly nitrogen and oxygen, air acts as a key reactant in the process, combining with natural gas to produce the compound.
- Catalysis: To speed up the chemical reaction between natural gas and air, a catalyst, often iron-based, is employed.
- Pressure: The production process demands high pressures, which are achieved using pumps or compressors to facilitate the reaction.
- Temperature: High temperatures are essential for the chemical reaction, achieved through heaters to create the optimal conditions for ammonia synthesis.
Once all the necessary ingredients are gathered, and the equipment is set up, the ammonia production process commences by mixing natural gas and air. This mixture is then subjected to high temperatures and pressures inside a reactor, with the catalyst and heater playing crucial roles. The result is the formation of ammonia and water through the chemical reaction.
The ammonia is later separated from the water and subjected to distillation for purification, removing any impurities and contaminants. Once purified, the ammonia is carefully stored and transported to its intended destination for final use.
The Haber-Bosch process follows a well-defined series of stages to produce ammonia, and let’s explore them in more detail:
1. Preparing the Feedstock:
To initiate the ammonia production process, the first crucial step involves obtaining natural gas, which serves as the feedstock. This natural gas is derived from underground sources and carefully processed to eliminate any impurities and contaminants.
2. Mixing Natural Gas and Air:
With the purified natural gas ready, it is combined with air in specific proportions. The presence of air is vital as it acts as a necessary component for the chemical reaction that leads to ammonia production.
3. Applying Heat and Pressure:
The mixture of natural gas and air is then introduced into a reactor, where it undergoes a controlled environment of high temperatures and pressures. Achieving these conditions involves the use of heaters and pumps or compressors.
4. The Chemical Reaction:
Inside the reactor, the magic happens – the natural gas reacts with air to yield both ammonia and water. The chemical reaction is expedited by employing an iron catalyst, which significantly accelerates the process.
The reactor plays a vital role in the Haber-Bosch process, where ammonia production takes place. This closed vessel is designed to facilitate the chemical reaction by mixing natural gas with air at high temperatures and pressures, ultimately yielding ammonia and water.
The size and capacity of the reactor depend on the desired ammonia output, ranging from small laboratory-scale reactors to large industrial ones. Moreover, reactors can be tailored to different shapes and designs, catering to specific process requirements.
Ensuring the reactor’s efficiency and safety is paramount in the Haber-Bosch process. It must be meticulously designed and constructed to withstand the high temperatures and pressures required for the chemical reaction. Additionally, appropriate safety measures must be incorporated to prevent any potential accidents.
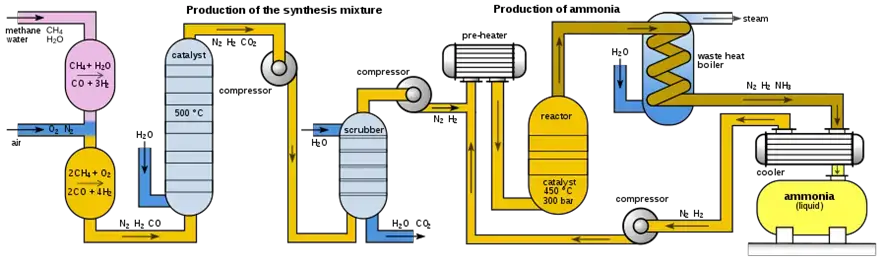
5. Ammonia separation and purification:
Once ammonia is produced, the next step is its separation and purification. The separation process involves heating the mixture to a temperature where ammonia evaporates, leaving water at the bottom of the vessel. The evaporated ammonia is then condensed, resulting in pure liquid ammonia droplets.
For purification, various chemical and physical methods like fractional distillation, filtration, and precipitation are employed to eliminate impurities and contaminants.
6. Ammonia storage and transport:
Finally, the purified ammonia is carefully stored and transported to its intended destination for its final application. Ammonia production plants typically follow this sequence of steps, ensuring a steady supply of this essential compound for various industrial and commercial uses.

Measures to Reduce Environmental Impact during Ammonia Production
It’s crucial to recognize that ammonia production can have significant environmental consequences, particularly due to the emission of nitrogen dioxide, a potent greenhouse gas. Additionally, the process demands substantial energy consumption, further contributing to climate change concerns.
To address these environmental challenges and promote sustainability, companies involved in ammonia production must take proactive measures. Some effective strategies to reduce the environmental impact include:
- Embrace Renewable Energy: Shifting to renewable energy sources, such as solar or wind power, to fulfill the energy requirements of the ammonia production process can greatly reduce carbon emissions and lower the overall environmental footprint.
- Greenhouse Gas Recycling: Implementing technologies to capture and recycle greenhouse gases produced during ammonia production can significantly curtail emissions, mitigating their impact on the environment.
- Carbon Capture and Storage: Adopting carbon capture and storage technologies enables the trapping of greenhouse gases, preventing their release into the atmosphere and effectively reducing the ammonia production process’s overall carbon footprint.
- Sustainable Production Techniques: Exploring cleaner and more sustainable methods, like ammonia production from biomass, can offer a greener alternative to conventional processes. Utilizing organic waste or biogas as feedstock not only reduces dependence on fossil fuels but also contributes to environmental preservation.
By integrating these environmentally conscious measures, ammonia producers can play a vital role in safeguarding the planet and advancing sustainable practices within their industry.
Conclusion
In summary, ammonia production is a chemical process that combines natural gas and air using high temperatures and pressures. While ammonia is essential and versatile, its production can harm the environment. To be more eco-friendly, companies making ammonia should take actions to lessen their impact and support sustainability.